A lithium-ion battery (LIB) is an indispensable energy storage device that has been extensively studied due to the accelerating evolution of environmentally friendly electric vehicles and portable electronics. Our recent studies discovered the ultrafast lithium-ion storage system of T phase niobium oxide (T-Nb2O5) and extensively examined the sophisticated mechanism in the charge/discharge processes; the detailed structural changes in delithiated/lithiated states of T-Nb2O5, vibrational characteristics and dynamics of Li ion migration have been systematically resolved by the density functional based calculations. We emphasized the charge storage mechanism and clarified that the loosely packed 4g layers in T-Nb2O5 can minimize the structural changes and are capable of stabilizing lithium ion adoptions, which is responsible for the excellent LIB durability. Also, the ultrafast storage of lithium ions in T-Nb2O5 corresponds to their low diffusion barriers and appropriated adsorption energies in 4g positions on the 2D void formed by the Nb-O bonds. Those findings not only discover the novel materials used for stable and highly efficient LIB, but also provide useful information for the better understanding of intercalation chemistry for those oxide-based materials applicable to the fast-growing market of power storage devices.
Mechanistic study of lithium diffusions and related vibrations in T-Nb2O5
The high-power energy storage system of lithium-ion batteries (LIB) has drawn considerable attention recently due to the fast growing adoption of electronic equipment and vehicles. One of the most critical challenges suffered by LIB technology is to find the promising electrodes that can efficiently store and stably release the energy with excellent durability. Numerous materials are promising candidates for LIB anodes, including carbonaceous nanomaterials, semiconductors, and transition metal oxides. Among them, T phase niobium oxide (T-Nb2O5) has been extensively studied because of its large capacity, high working potential, and, most importantly, layered crystal structure. In this selected work, we have utilized the vibrational analysis and dynamic calculations to resolve the detailed mechanism, for the better understanding of chemical insights beneath the ultrafast Li ion storage of T-Nb2O5 and the rational design of niobium oxide-based anodes to promote LIB performance.
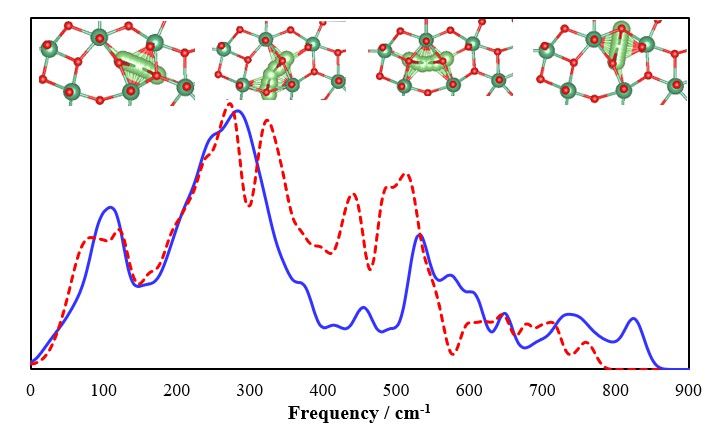
Crystal structures of unlithiated and lithiated T-Nb2O5 have been initially optimized to represent the anodic materials in the discharged and charged states, respectively. The 4g layer in unlithiated T-Nb2O5 with loose Nb-O bonds shows a large number of voids, which are preferential sites for Li ion accommodation to ease the stress of structural changes and repulsion from positively charged metallic elements (Figure 1). Accordingly, lithiated T-Nb2O5 has Li ions fully adsorbed on those voids in the 4g layer; the computed adsorption energies are in the range of -3.11 ~ -3.53 eV for single Li ion adsorption, and the averaged adsorption energy is -2.38 eV for the full adsorption. Based on the optimized structures, we further analyzed the vibrations of lithiated and unlithiated T-Nb2O5 to understand the structural evolution in the charge and discharge processes.
The irreducible representation of vibrations for Raman active modes
Unlithiated: ΓRaman=28Ag+28B1g+14B2g+14B3g
Lithiated: ΓRaman=32Ag+32B1g+16B2g+16BB3g
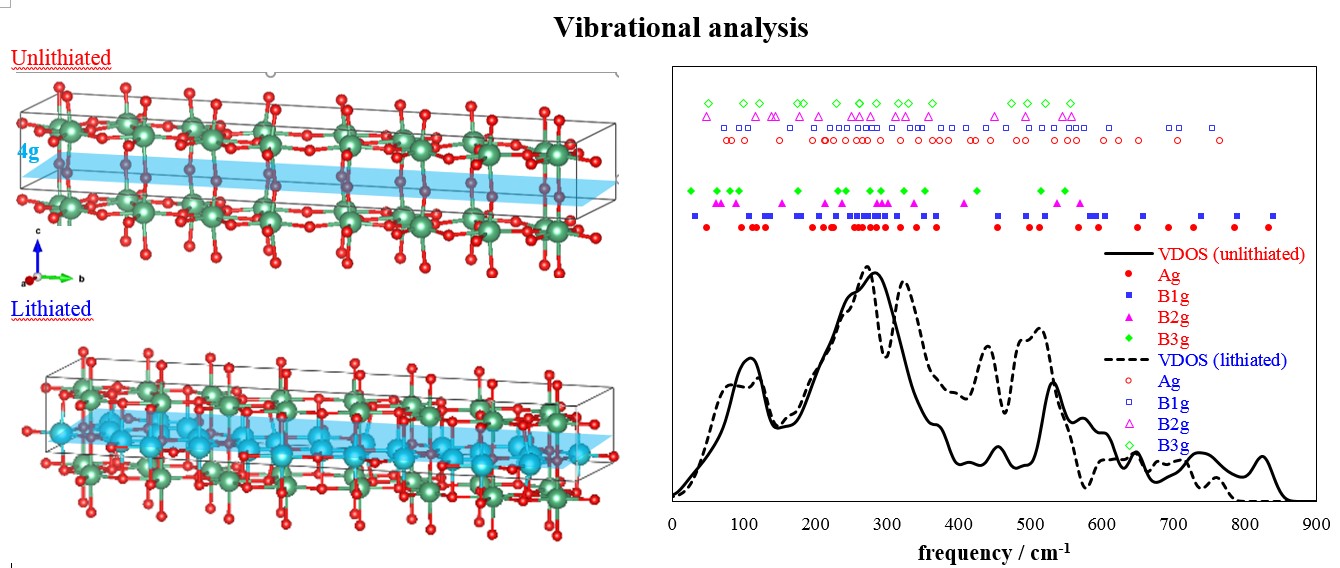
Figure 1.Structures and vibrations of unlithiated and lithiated Nb2O5.
Additionally, the continuous distribution of vibrational density of states (VDOS), resulting from phonon dispersion and integrating at the Γ-point, has been examined. Those vibrations can be grouped in the low (100-300 cm-1), mid (300-650 cm-1) and high (>650 cm-1) frequency regions, which correspond to Nb-O stretching modes perpendicular to the 4g layer, parallel to the ab plane and O-Nb-O bending modes (Figure 1), respectively. On lithiated T-Nb2O5 (charging process), high-frequency vibrations diminished obviously, and more vibrations appeared in the mid-frequency region; low-frequency vibrations showed limited changes. The noticeable changes in high and mid-frequency vibrations, confirmed with Raman spectroscopy, are direct evidence of Li ion adsorption in the loosely packed 4g layer during the charging process.
The charge dynamics have been studied by examining the migration of a Li ion from one site to another in T-Nb2O5. According to the vibrational analysis, Li ions preferentially adsorb on the 4g plane; it is thus concluded that the migration pathways of Li ions are sticky on the plane. Additionally, the migration can be categorized into four simple path topologies in terms of the neighboring Nb–O bonding structures; as shown in Figure 2, Li ions migrate to bond with edge-shared O in pathways 1 and 4 and to the corner-shared ones in pathways 2 and 3. Since the void size, that is, the distance between adjacent ab planes, is large enough (approximately 4 Å) to retard the migration, the energetic barriers for Li-ion migration are mainly obstructed by the nonbridged oxygen atoms located on the 4g plane. The computed barriers in those migration pathways are rather small (<0.4 eV), indicating that Li ions can easily diffuse on the 4g plane. Also, the migrations are all on the 4g plane, implying that the migrations of Li ions have directionality. After careful emphasis of the migration topologies and barriers, our result has identified the evident merits responsible for fast Li-ion transport in the novel T-Nb2O5; those unique features distinguish T-Nb2O5 from other classical intercalation-type materials and account for the excellent electrochemical performance.
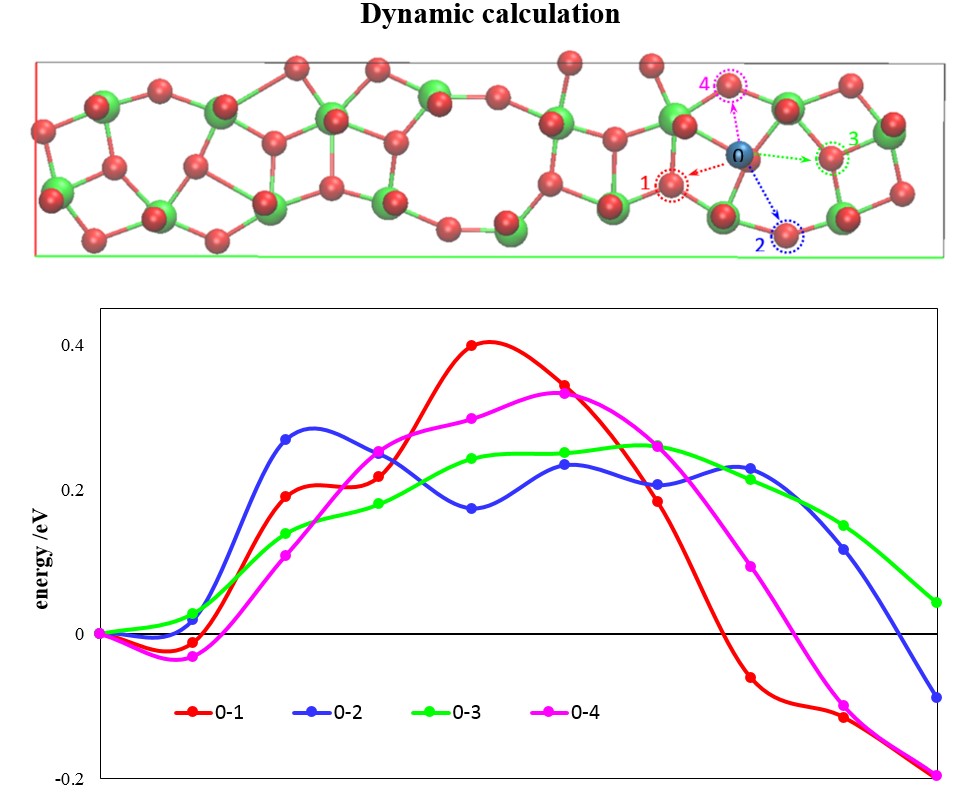
Figure 2. Lithium diffusion pathways and related energetics in T-Nb2O5.
In order gain a comprehensive understanding of the energy storage mechanism of T-Nb2O5 used in LIB technology, our study extensively examined the structural and vibrational characteristics of unlithiated and lithiated models, well verified with the experimental Raman spectroscopy, and analyzed the migrating dynamics of Li ions. Our results identified the preferential adsorption sites and migration pathways located on the loosely packed 4g layer that can stabilize the structure and assist the ultrafast charge/discharge processes, thus resulting in a superior LIB performance. It is believed that our work elucidates the key concepts for the mechanistic understanding and can further be utilized to rationally optimize niobium oxide based anodes in LIB application.
Source:
https://pubs.acs.org/doi/abs/10.1021/jacs.7b03141
https://pubs.acs.org/doi/abs/10.1021/acs.nanolett.9b00179
https://doi.org/10.1016/j.nanoen.2021.106398








